Medovate Ltd, an award-winning medical device development company and JEB Technologies Ltd, an established leader in end-to-end medical device design and manufacturing, have recently integrated their management and expertise to drive innovation in the dynamic medical device development industry. Medovate and JEB Technologies have combined their strengths across the full medical device development pipeline, from concept design, development and manufacturing, through to regulatory approvals and onward commercialisation.
 Medovate works collaboratively with clinical innovators and the NHS to bring new medical devices to market in the UK and internationally in order to improve patient safety and care. With a specialist-built company headquarters in Mildenhall incorporating a Class 8 cleanroom, JEB’s design, development and manufacturing capabilities include injection moulding, CNC machining, electronic assembly, in-house tooling, stamping, laser marking and welding, multi spindle turning and ultrasonic welding.
Medovate works collaboratively with clinical innovators and the NHS to bring new medical devices to market in the UK and internationally in order to improve patient safety and care. With a specialist-built company headquarters in Mildenhall incorporating a Class 8 cleanroom, JEB’s design, development and manufacturing capabilities include injection moulding, CNC machining, electronic assembly, in-house tooling, stamping, laser marking and welding, multi spindle turning and ultrasonic welding.
Following the two companies joining forces, Stuart Thomson, Vice President of Business Development, said, “This is a significant step forward for the medical device industry in the UK’s eastern region, leveraging our organisations’ collective strengths and expertise, along with respective medical technology pipelines, to accelerate the development of innovative UK medical devices and launch them successfully around the world. Medovate’s team working seamlessly with JEB Technologies will drive innovation development to positively impact healthcare globally.”
The two company’s portfolios include SAFIRA® (SAFer Injection for Regional Anaesthesia), designed to revolutionise standard regional anaesthesia practice and promote patient safety. Typically, regional anaesthesia procedures require two operators, whereas the SAFIRA system enables a single operator to conduct the whole regional block process using either a foot pedal or a palm operator to control infusion and aspiration themselves, facilitating consistent, controlled delivery. With its built-in safety mechanism, the device stops injection at a specific threshold, reducing the chance of nerve injury.
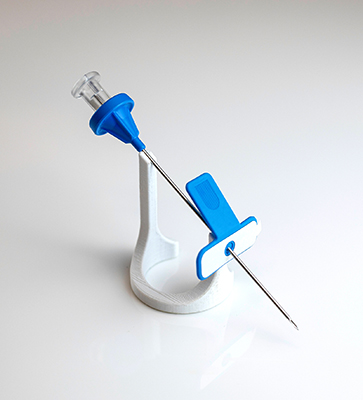
Another novel device is CamPROBE, a safe, simple and cost effective transperineal prostate biopsy device. This consists of a stainless-steel cannula, an integrated coaxial needle and an adhesive attachment base. The needle is inserted at two entry points on either side of the perineum and CamPROBE is then advanced to the prostate area under ultrasound guidance and simultaneous local anaesthetic delivery. The integrated needle is then removed and the cannula can be used as an access sheath for biopsies. With only two body puncture points required, along with the simultaneous infusion of anaesthetic, the CamPROBE device reduces risks whilst providing patient comfort benefits during prostate biopsies.
 T +44 (0)1223 901991
T +44 (0)1223 901991
sales@medovate.co.uk
https://jeb-technologies.com
https://www.medovate.co.uk

